2. Program Study of Marine Science, University of Sriwijaya, Kampius Inderalaya, Ogan Ilir, South Sumatra, Indonesia
3. Program Study of Fisheries, University of Diponegoro, Kampus Tembalang, Semarang,West Java, Indonesia
4. Program Study of Marine Science, University of Diponegoro, Kampus Tembalang, Semarang,West Java, Indonesia
 Author
Author  Correspondence author
Correspondence author
International Journal of Marine Science, 2015, Vol. 5, No. 53 doi: 10.5376/ijms.2015.05.0053
Received: 07 May, 2015 Accepted: 08 Aug., 2015 Published: 17 Sep., 2015
Hendri M.1,, Darmanto J. S., Prayitno B. and Radjasa O.K., 2015, Antibacterial Potential Screening of Halimeda sp on Some Types of Pathogenic Bacteria, International Journal of Marine Science, 5(53): 1-6 (doi: 10.5376/ijms.2015.05.0053)
The study was conducted as a test to determine the effectiveness of Halimeda sp seaweed extract on the growth of some types of pathogenic bacteria. Seaweeds extracted consist of four (4) types which include: Halimeda macrophysa, Halimeda gracillis, Halimeda Opuntia and Halimeda renschi. While the types of pathogenic bacteria used were (Salmonella typhi, Staphylococcus aureus, Escherichia coli and Bacillus subtilis). This study uses methanol in the ratio of 1: 1 (v/v) and were observed for 48 hours. The test results showed that the extract of Halimeda sp is effective as antibacterial pathogen. Phytochemical test showed the presence of steroid and saponin compounds.
1 Introduction
Seaweed is one of important marine commodities that have high economic value for export. Currently seaweed has been developed by means of cultivation. This activityy is carried out by various parties such as companies, governments, and fishermen community. The benefits of this plant are commonly known as product of food, beauty, medicines and others (Aslan, 1998; Anggadireja et al., 2006).
Some marine organisms, especially from the class of marine algae, have the ability to produce chemical compounds that are not found or rarely found in organisms that live on land (Nybakken, 1993). Some types of marine biota synthesize and store toxic compounds called marinetoxin on parts of his body and released into the environment (Djapiala et al., 2013; Anggadireja et al., 2006). These compounds are secondary metabolites which are used as a defense and to preserve life, to avoid interference from predators. These compounds have pharmacological activity, so it is possible to be developed (Paul and Fenical, 1983; Paul and Puglisi, 2004; Paul and Fenical, 1984; Paul and Van Alstyne, 1988).
Halimeda is a marine plant that has green leaves and is one type of green algae group. Halimeda has the ability to produce bioktif substances for antifouling. The active substance produced for biofouling is known as halimedatrial and halimedatetraasetat. Halimedatrial is diterpenoid that yet trialdehyde, known as the major secondary metabolite in six species of algae containing calcium Halimeda (Paul and Fenical, 1983; Paul and Fenical, 1984; Paul and Puglisi, 2004; Kumar et al., 2010; Bachtiar et al., 2012; Paul, 1987).
Seaweed, primarily from the group Halimeda sp has the ability to issue a secondary metabolite in the process of metabolism to defend themselves against predators and pests. The active ingredients released by Halimeda are very effective to prevent attacks of predators and bacteria (antifouling). Halimedatrial and halimedatetraasetate a bioactive compounds contained in seaweed (Halimeda sp) (Paul and Fenical, 1983; Paul and Fenical, 1984; Paul and Fenical, 1986; Paul, 1987; Atmadja, 1992; Paul and Van Alstyne, 1992).
The ability of algae to produce halogenated secondary metabolites that act as bioactive compounds might happen, because the environmental conditions such as high salinity or will be used to defend themselves from the threat of predators. In the last decade, a variety of structures of bioactive compounds that very unique from red algae have been isolated. However, utilization of bioactive ingredients from algae has not been done. Based on the biosynthesis process, marine algae are rich in compounds derived from the oxidation of fatty acids called oxylipin. Through these compounds various types of secondary metabolites are produced (Paul and Fenical, 1983; Paul and Fenical, 1986; Hay and Fenical, 1988; Paul and Puglisi, 2004; Hay, 1996; Karthikaidevi et al., 2009; Kolsi et al., 2015).
Halimeda chemically able to produce diterpenoid metabolites halimedatrial and Halimeda tetra acetate at various concentrations. This metabolite has been observed to play a role in chemical defense against herbivores, based on their chemical structure and biological activity. Halimedatrial more effective than halimedatetraasetat in marine algae defense system to repel natural enemies (Paul and Fenical, 1983; Paul and Fenical, 1984; Paul and Fenical, 1986; Paul and Van Alstyne, 1988; Paul and Puglisi, 2004)
2 Material and Methods
Materials
Seaweed Halimeda sp, collected from the waters of the Gulf of Lampung. Sampling was carried out in June - July 2014 and analyzed at the Marine Biological Laboratory Faculty of University of Sriwijaya, Basic Chemistry and Biotechnology Laboratory LIPI Cibinong.
Samples seaweed washed with running water and rinsed with sterile water and cut into small pieces. Subsequently dried and crushed made flour. Halimeda sp extracted with methanol, evaporated with a rotary evaporator. Extracts of secondary metabolites identified by thin-layer chromatography (TLC) or thin-layer chromatography (TLC). Dry extract sample dissolved in methanol is used as the test solution, then spotted by 5 mL of test solution and standard solution on the plates of silica gel GF 254 as the stationary phase. Put the plates into the chromatography vessel that has been saturated with mobile phase consisting of a mixture of Chloroform-methanol (10: 1) v/v. Elution until the upper limit of the stationary phase plate. Identification chromatography with UV light of 254 nm, and then sprayed with cerium sulfate reagent. Then dried and viewed with UV 254 nm.
Other materials used are pathogenic bacterial culture types S.typhi, S. aureus, E. coli and B.subtilis obtained from laboratory Basic Chemistry and Biotechnology LIPI Cibinong Bogor. Media for the pace of the bacteria used are nutrient agar (NA) and liquid nutrient broth (NB).
The tools used include blenders, autoclaves, incubators, distillator, pH meter, ose needle, micropipette, magnetic stirrer, micrometers, shaker, hot plates and oven.
Antimicrobial Materials Selection
At this stage, the analysis of water content materials is conducted (Apriyantono et al., 1989) and the selection of materials using solvent extraction of water and testing activities by the agar diffusion method.
Extraction of materials
The extraction step includes the destruction of material, the addition of water at a ratio of materials and water 1:1, 1:2, 1:3 (w/v), then filtering treatment. The filtrate obtained is sterilized.
b. Testing antimicrobial activity by agar diffusion method (Wolf and Gibbons, 1996). Nutrient Agar (NA) which has been sterilized cooled to a temperature of 50o C in a water bath. Each bacterial culture was aged 24 hours at a concentration of 107-108 cells per mlk inserted into the NA of 40 uL for every 20 ml of NA. Subsequently made to the cup with a thickness of 4-5 mm.
Then put the paper disc that has been dipped in each extract Halimeda. Subsequently incubated at 370 C for 48 hours. Then observed the presence of inhibitory and in measuring the diameter of inhibition (in mm) using a micrometer measuring tool. This stage is carried out with two replications.
3 Result and Discussion
Result
Antibacterial Test Results
Halimeda sp crude extract was tested by using four (4) types of pathogen bacteria (S.typhi, S. aureus, E. coli, B. subtilis) with treatment four (4) types of Halimeda sp extracts which include: H.macrophysa, H. incrassata, H.opuntia and H.renschi. This test is done observation for 48 hours. In general, the effect of this extract is significant to the growth of these bacteria (see Table 1).
|
Table 1 Methanol Extract Antibacterial Test Result from Halimeda sp (48 hours)
|
Phytochemicals Test Results
The phytochemical test results showed extracts H.renschi and H. gracillis containing steroids and saponins compounds, while alkaloids, terpenoids, tannins and flavonoids are not contained in the extract (Table 2).
KLT Test Result
Furthermore, each extract was analyzed with TLC plate, TLC Results showed suspected stain patterns potentially contain secondary metabolites with the invisibility of the dominant pattern fluorescent stain under UV light but the compound is not pure and there are still impurities. Isolation and purification are still needed to determine the type of the active compound (Figure 1).
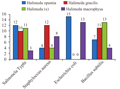
The test results on the four (4) types of Halimeda sp are extracted on the growth of E. coli bacteria showed that extracts of H.opuntia and H. macrophysa which has a significant influence with a diameter of between 13-15 mm. This shows that the extract has a Halimeda extract inhibition against the bacteria E. coli. While H.gracillis and H.renschi no effect. The pattern of growth can be seen in Figure 2.
 Figure 2 Results of Antibacterial Test Methanol Extracts of the E. coli bacteria on a 48 hours observation (A. H.opuntia, B H.gracillis, C. H renschi and D. H macrophysa) |
To test the growth of S. aureus on the four Halimeda sp seaweed extract shows have influence with a diameter of 4-12 mm. The largest to the smallest diameter is the extract of H. gracillis, H. macrophysa, H. renschi, and H. Opuntia. This means that all sample extracts have the ability/inhibition of the growth of bacteria. The highest inhibition owned by extracts of seaweed species H. gracillis with 12 mm. While the lowest inhibitory owned by H. renschi and H. opuntia with inhibition of 4 mm. The pattern of growth can be seen in Figure 3.
 Figure 3 Antibacterial Test Results methanol extract against S. aureus bacteria at 48 hours observation (A. H. opuntia, B H.gracillis, C. H.renschi and D. H macrophysa) |
Halimeda sp extract test results against bacterial growth related B. subtilis shows that extracts of Halimeda have influence with diameter about 4-13 mm. The highest inhibition by 13 mm at H.renschi extracts, whereas inhibition of the lowest in the extract of H. macrophysa with a diameter of 4 mm (see Figure 4).
 Figure 4 Results of Antibacterial Test Methanol Extracts of the bacteria B. subtilis at 48 hours observation (A. H.opuntia, B. H.gracillis, C. H.renschi and D. H macrophysa) |
The fourth extract Halimeda also tested for bacterial growth S.typhi. The test results showed that the extract had an influence with a diameter of 3-12 mm. The most high-power inhibitor is owned by H. opuntia with diameter of 12 mm, then H.renschi with 11 mm and H. gracillis with a diameter of 10 mm. While the lowest seaweed extract is H. macrophysa with a diameter of only 3 mm. The pattern of growth can be seen in Figure 5.
 Figure 5 Results of Antibacterial Test Methanol Extracts against bacteria S. Typhi in observation 48 hours (A. H.opuntia, B H.gracillis, C. H.renschi and D. H.macrophysa) |
These results indicate that the aforementioned extraction have a fairly good inhibitory to the growth of pathogenic bacteria such as S. typhi, S.aureus, B.subtilis, E.coli bacteria. Anti-bacterial activity demonstrated in this study is active. The types of extracts based on test results of the highest H.opuntia active are in E. coli bacteria, the highest H. gracillis extract in S. aureus, the highest H. macrophysa extract in E.coli bacteria. While the highest extract of this type of H.renschi is in B. subtilis bacteria (Figure 6).
In addition each extract has anti-bacterial capabilities that varies depending on the type of extracts and bacterial strains. This means that the zone of inhibition showed antimicrobial activity against pathogens bacteria is varied. The ability of Halimeda sp extract to inhibits the growth of bacteria is also influenced by the test bacterial cell wall. (Fardiaz, 1983) states that the positive and gram-negative bacteria have different cell wall sensitivity against physical treatment, enzymes and antibodies (Atmadja, 1992; Izzati, 2007; Shanab, 2007).
Discussion
The bacteria used in this study is a gram-negative and gram-positive, was able to be inhibited by the extract of Halimeda sp.Gram negative bacteria have a better resistance to anti-microbial compounds compared with gram-positive. (Branen and Davidson, 1983) states that gram-negative bacteria have a selection system against foreign substances at the lipopolysa- ccharide layer (Davidson et al., 2005). While (Pelczar and Chan. 1986) states positive gram bacterial cell wall structure is relatively simpler making it easier for antimicrobial compounds to enter the cell and find a target to work. The structure of the cell wall of gram-negative bacteria are relatively more complex, triple layers namely the outer layer in the form of lipoproteins, the middle layer in the form of lipopolysaccharide and peptidoglycan layer.
The phytochemical test results showed H.renschi and H. gracillis extracts contain steroid and saponins compounds, while alkaloids, terpenoids, tannins and flavonoids are not contained in the extract. In accordance with the phytochemical test results that containing steroids compound, then it is consistent with the NMR test results which found the active compounds in the form of β-sitosterol in the n-hexane solvent. β-sitosterol included in one type of steroid. In addition to the discovery of β-sitosterol, the extract was also found that the oleat acid compound is part of primary metabolite (Hendri, 2015; Anam, 1999; Shanab, 2007).
The phytochemical test results on other research state that extracts of Caulerpa sp, Euchema sp, Gracilaria sp and Sargassum sp contain alkaloids, flavonoids, steroids, triterpenoids and tannins (Siregar et al., 2012). Other phytochemical test results state that bioactive steroid compounds always found in a variety of phytochemical test (Siregar et al., 2012; Alamsyah et al., 2014), whereas the saponin compound in (Siregar et al., 2012) study is not found at all of the four seaweed extract tested, whereas in the study of (Firdaus., 2008; Alamsyah et al., 2014), saponins can be found.
(Kolanjinathan et al., 2009) reported the discovery of several compounds that are bioactive metabolites derived from several types of seaweed that is; brominated, aromatic, nitrogen-heterocyclic, sterols, protein, and polysaccharide sulfate. Results of another study states that Sargassum sp has potential as an antioxidant. Specifically, this plant contains phlorotanin, a polyphenol that is not found in other plants or seaweed. These compounds have proven capable of inhibiting lipid peroxidation and free radical activity. S. duplicatum contain alkaloids, saponins, tannins, steroids and glycosides with phlorotanin levels from 9.2822 to 37.3693 mg/g. Retention time fraction extract: 0.97; 0.75, and 0.46, and efficiency of anti-radical is 11264.54 (Firdaus., 2008).
Conclusions
Results of the study of the effectiveness of extracts of Halimeda sp against pathogens is have antibacterial activity against bacteria of S.typhi, S. aureus, E. coli and B. subtilis and has effectiveness to decrease the amount of pathogenic bacteria. The phytochemical test result showed steroid and saponins compounds. While the TLC test results indicate the potential of the compound, although not pure. Halimeda sp extracts have antimicrobial. However, further research is needed to determine the compounds that exist and chemical structure. Environmental and geographical factors need to be done to see the influence on the type and content of the active compound.
References
Alamsyah H.K., Widowati I., and Sabdono A., 2014, Aktivitas antibakteri ekstrak rumput laut sargassum cinereum (jg agardh) dari perairan pulau panjang jepara terhadap bakteri escherichia coli dan staphylococcus epidermidis, Journal of Marine Research, 3: 69-78
Anam K. (1999) Telaah Kandungan Kimia Caulerpa sertularioides (Vahl) C. Agardh, Caulerpaceae, Bandung, Sekolah Farmasi ITB.Tesis.
Anggadireja J., Zatnika. A., H.Purwoto, and Istini. (2006) Rumput Laut, Pembudidayaan, Pengolahan Potensial dan Pemasaran Komoditas Perikanan Potensial., Jakarta, Penebar Swadaya.
Apriyantono A., Fardiaz D., Puspitasari N.L., Sedarnawati, and Budiyanto S. (1989) Analisis Pangan, Bogor, PAU Pangan dan Gizi. IPB Press.
Aslan L.M. (1998) Rumput Laut, Yogyakarta, Kanisius.
Atmadja W.S. (1992) Potensi dan spesifikasi jenis rumput laut di Indonesia. IN ADI (Ed.) Prosiding Temu Karya Ilmiah Teknologi Pasca Panen Rumput Laut Puslitbang Perikanan. Jakarta Balai Litbang Perikanan.
Bachtiar S.Y., Tjahjaningsih W., and Sianita N., 2012, Pengaruh ekstrak alga cokelat (sargassum sp.) terhadap pertumbuhan bakteri escherichia coli. Effect of algae brown (sargassum sp.) extract against bacterial growth of escherichia coli, Journal of Marine and Coastal Science, 1: 53-60
Branen A.L., and Davidson P.M. (1983) Antimicrobials in foods, Marcel Dekker, Inc.
Davidson P.M., Sofos J.N., and Branen A.L. (2005) Antimicrobials in food, CRC press.
http://dx.doi.org/10.1201/9781420028737
Djapiala F.Y., Montolalu L.A., and Mentang F., 2013, Kandungan Total Fenol dalam Rumput Laut Caulerpa racemosa yang Berpotensi Sebagai Antioksidan, JURNAL MEDIA TEKNOLOGI HASIL PERIKANAN, 1
Fardiaz S. (1983) Keamanan Pangan Bogor, IPB Press.
Firdaus. M. (2008) Penapisan Fitokimia, Penentuan Kadar Phlorotanin dan Uji Aktivitas Antioksidan Ekstrak Rumput Laut Coklat (Sargassum duplicatum). IN UGM, P. S. (Ed.) Prosiding Semnaskan UGM.Bidang Bioteknologi. Yogyakarta.
Hay M.E., 1996, Marine chemical ecology: what's known and what's next?, Journal of Experimental Marine Biology and Ecology, 200: 103-134
http://dx.doi.org/10.1016/S0022-0981(96)02659-7
Hay M.E., and Fenical W., 1988, Marine plant-herbivore interactions: the ecology of chemical defense, Annual review of ecology and systematics: 111-145
http://dx.doi.org/10.1146/annurev.es.19.110188.000551
Hendri M. (2015) Eksplorasi Senyawa Bioaktif Rumput Laut Halimeda renschi dan Halimeda grasillis di Perairan Teluk Lampung Sebagai Sumber Senyawa Antibakteri. Semarang,Jawa Tengah. Indonesia, Program Studi MSDP Universitas Diponegoro,Disertasi.
Izzati M., 2007, Skreening potensi antibakteri pada beberapa spesies rumput laut terhadap bakteri patogen pada udang windu, BIOMA, 9: 62-67
Karthikaidevi G., Manivannan K., Thirumaran G., Anantharaman P., and Balasubaramanian T., 2009, Antibacterial properties of selected green seaweeds from Vedalai coastal waters; Gulf of Mannar marine biosphere reserve, Global J Pharmacol, 3: 107-112
Kolanjinathan K., Ganesh P., and Govindarajan M., 2009, Antibacterial activity of ethanol extracts of seaweeds against fish bacterial pathogens, Eur Rev Med Pharmacol Sci, 13: 173-177
Kolsi R.B.A., Frikha D., Jribi I., Hamza A., Fekih L., and Belgith K., 2015, Screening of antibacterial and antifongical activity in marine macroalgae and magnoliophytea from the coast of Tunisia, International Journal of Pharmacy and Pharmaceutical Sciences, 7
Kumar S.S., Kumar Y., Khan M., and Gupta V., 2010, New antifungal steroids from Turbinaria conoides (J. Agardh) Kutzing, Natural product research, 24: 1481-1487
http://dx.doi.org/10.1080/14786410903245233
Nybakken J.W. (1993) Marine Biologi., Harper Collins College publisher. Third Edition. .
Paul V.J., 1987, Feeding deterrent effects of algal natural products, Bulletin of marine science, 41: 514-522
Paul V.J., and Fenical W., 1983, Isolation of halimedatrial: chemical defense adaptation in the calcareous reef-building alga Halimeda, Science, 221: 747-749
http://dx.doi.org/10.1126/science.221.4612.747
Paul V.J., and Fenical W., 1984, Novel bioactive diterpenoid metabolites from tropical marine algae of the genus Halimeda (Chlorophyta), Tetrahedron, 40: 3053-3062
http://dx.doi.org/10.1016/S0040-4020(01)82430-3
http://dx.doi.org/10.1016/S0040-4020(01)91301-8
Paul V.J., and Fenical W., 1986, Chemical defense in tropical green algae, order Caulerpales, Mar. Ecol. Prog. Ser, 34: 157-169
http://dx.doi.org/10.3354/meps034157
Paul V.J., and Puglisi M.P., 2004, Chemical mediation of interactions among marine organisms, Natural product reports, 21: 189-209
http://dx.doi.org/10.1039/b302334f
Paul V.J., and Van Alstyne K.L., 1988, Chemical defense and chemical variation in some tropical Pacific species of Halimeda (Halimedaceae; Chlorophyta), Coral Reefs, 6: 263-269
http://dx.doi.org/10.1007/BF00302022
Paul V.J., and Van Alstyne K.L., 1992, Activation of chemical defenses in the tropical green algae Halimeda spp, Journal of Experimental Marine Biology and Ecology, 160: 191-203
http://dx.doi.org/10.1016/0022-0981(92)90237-5
Pelczar M.J., and Chan.. E.C.S. (1986) Dasar-dasar mikrobiologi I, Jakarta. Shanab S.M., 2007, Antioxidant and antibiotic activities of some seaweeds (Egyptian isolates), Int J Agric Biol, 9: 220-225
Siregar A.F., Sabdono A., and Pringgenies D., 2012, Potensi Antibakteri Ekstrak Rumput Laut Terhadap Bakteri Penyakit Kulit Pseudomonas aeruginosa, Staphylococcus epidermidis, dan Micrococcus luteus, Journal of Marine Research, 1: 152-160
Wolf C., and Gibbons W., 1996, Improved method for quantification of the bacteriocin nisin, Journal of applied bacteriology, 80: 453-457
http://dx.doi.org/10.1111/j.1365-2672.1996.tb03242.x
. PDF(323KB)
. FPDF(win)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Hendri M.
. Darmanto J. S.
. Prayitno B.
. Radjasa O.K.
Related articles
. Antibacterial
. Bacterial Pathogens
. Halimeda sp
. Seaweed
Tools
. Email to a friend
. Post a comment